The Effects of Prunus spinosa L. Flower Extracts, Model Polyphenols and Phenolic Metabolites on Oxidative/Nitrative Modifications of Human Plasma Components with Particular Emphasis on Fibrinogen In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Extracts Preparation
2.2. Reference Standards of Model Polyphenols and Phenolic Metabolites
2.3. Synthesis of ONOO−
2.4. Antioxidant Activity in Human Plasma Model
2.4.1. Isolation of Blood Plasma and Preparation of Samples
2.4.2. Determination of 3-NT in Plasma Proteins
2.4.3. Determination of TBARS in Plasma
2.4.4. Determination of the NEAC of Plasma
2.5. Activity against Oxidative/Nitrative Modifications of Human Fibrinogen
2.5.1. Isolation of Fibrinogen from Blood Plasma
2.5.2. SDS PAGE Analysis
2.5.3. Western Blot Analysis
2.5.4. C-ELISA of the ONOO−-Induced 3-NT Formation
2.5.5. Fluorometric Analysis of the ONOO−-Induced Tryptophan Residue Modifications
2.6. Statistical Analysis
3. Results
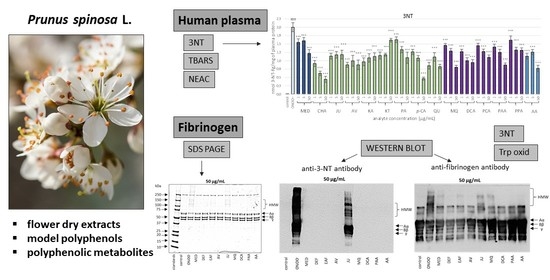
3.1. Protective Effects on Human Plasma Components against the ONOO−-Induced Oxidative Stress
3.2. Protective Effects against Oxidative/Nitrative Modifications of Fibrinogen
3.2.1. SDS-PAGE Analysis of the ONOO−-Induced Changes in the Isolated Fibrinogen
3.2.2. Western Blot Analysis of the Isolated Fibrinogen with Anti-3-NT Antibody
3.2.3. Determination of the ONOO−-Induced 3-NT Formation in the Isolated Fibrinogen by C-ELISA
3.2.4. Determination of the ONOO−-Induced Modifications of Tryptophan Residues in the Isolated Fibrinogen
3.2.5. Influence of the Analytes on the ONOO−-Induced Modifications of Fibrinogen in Blood Plasma Matrix—Western Blot Analysis with Anti-Fibrinogen Antibody
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3-NT | 3-nitrotyrosine |
| AA | ascorbic acid |
| AV | avicularin (quercetin 3-O-α-L-arabinofuranoside |
| C-ELISA | the competitive enzyme-linked immunosorbent assay |
| CHA | chlorogenic acid |
| CVDs | cardiovascular diseases |
| CYE | Cyanidin chloride equivalents |
| DCA | 3-(3′,4′-dihydroxyphenyl)propionic acid (dihydrocaffeic acid) |
| DEF | diethyl ether fraction |
| dw | dry weight |
| EAF | ethyl acetate fraction |
| GAE | Gallic acid equivalents |
| H2O2 | hydrogen peroxide |
| HOCl | hypochlorous acid |
| HMW | high molecular weight |
| JU | juglanin (kaempferol 3-O-α-L-arabinofuranoside) |
| KA | kaempferol |
| KT | kaempferitrin (kaempferol 3,7-di-O-α-L-rhamnopyranoside) |
| LOOH | lipid hydroperoxides |
| MED | defatted methanol-water (7:3, v/v) extract |
| MQ | miquelianin (quercetin 3-O-β-D-glucuronopyranoside) |
| MW | molecular weight |
| NEAC | non-enzymatic antioxidant capacity of plasma |
| NO• | nitric oxide |
| ONOO− | peroxynitrite |
| O2•− | superoxide anion |
| OH• | hydroxyl radical |
| PA2 | proanthocyanidin A2 |
| PAA | 2-(3′,4′-dihydroxyphenyl)acetic acid |
| p-CA | p-coumaric acid |
| PCA | protocatechuic acid |
| PPA | 3-(4′-hydroxyphenyl)propionic acid |
| QU | quercetin |
| ROS | reactive oxygen species |
| SDS-PAGE | sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| TAC | total content of phenolic acids (HPLC-PDA) |
| TBARS | thiobarbituric acid-reactive substances |
| TFC | total flavonoid content (HPLC-PDA) |
| TPA | total proanthocyanidin content (n-butanol/HCl assay) |
| TPC | total phenolic content (Folin-Ciocalteu assay) |
| TPH | total phenolic content (HPLC-PDA-fingerprint) |
References
- D’Oria, R.; Schipani, R.; Leonardini, A.; Natalicchio, A.; Perrini, S.; Cignarelli, A.; Laviola, L.; Giorgino, F. The role of oxidative stress in cardiac disease: From physiological response to injury factor. Oxid. Med. Cell. Longev. 2020, 2020, 5732956:1–5732956:29. [Google Scholar] [CrossRef] [PubMed]
- Cervantes Gracia, K.; Llanas-Cornejo, D.; Husi, H. CVD and oxidative stress. J. Clin. Med. 2017, 6, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marrocco, I.; Altieri, F.; Peluso, I. Measurement and clinical significance of biomarkers of oxidative stress in humans. Oxid. Med. Cell. Longev. 2017, 2017, 6501046:1–6501046:32. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J. Protein oxidation and peroxidation. Biochem. J. 2016, 473, 805–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parastatidis, I.; Thomson, L.; Burke, A.; Chernysh, I.; Nagaswami, C.; Visser, J.; Stamer, S.; Liebler, D.C.; Koliakos, G.; Ischiropoulos, H.; et al. Fibrinogen beta-chain tyrosine nitration is a prothrombotic risk factor. J. Biol. Chem. 2008, 283, 33846–33853. [Google Scholar] [CrossRef] [Green Version]
- Nowak, P.; Zbikowska, H.M.; Ponczek, M.; Kolodziejczyk, J.; Wachowicz, B. Different vulnerability of fibrinogen subunits to oxidative/nitrative modifications induced by peroxynitrite: Functional consequences. Thromb. Res. 2007, 121, 163–174. [Google Scholar] [CrossRef]
- Martinez, M.; Cuker, A.; Mills, A.; Lightfoot, R.; Fan, Y.; Tang, W.H.; Hazen, S.L.; Ischiropoulos, H. Nitrated fibrinogen is a biomarker of oxidative stress in venous thromboembolism. Free Radic. Biol. Med. 2012, 53, 230–236. [Google Scholar] [CrossRef] [Green Version]
- Ariëns, R.A. Fibrin(ogen) and thrombotic disease. J. Thromb. Haemost. 2013, 11, 294–305. [Google Scholar] [CrossRef]
- Medeiros, R.; Sousa, B.; Rossi, S.; Afonso, C.; Bonino, L.; Pitt, A.; López, E.; Spickett, C.; Borthagaray, G. Identification and relative quantification of 3-nitrotyrosine residues in fibrinogen nitrated in vitro and fibrinogen from ischemic stroke patient plasma using LC-MS/MS. Free Radic. Biol. Med. 2021, 165, 334–347. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Lamuela-Raventos, R.M.; Moreno, J.J. Polyphenols, food and pharma. Current knowledge and directions for future research. Biochem. Pharmacol. 2018, 156, 186–195. [Google Scholar] [CrossRef]
- Koch, W. Dietary polyphenols-important non-nutrients in the prevention of chronic noncommunicable diseases. A systematic review. Nutrients 2019, 11, 1039. [Google Scholar] [CrossRef] [Green Version]
- Bijak, M.; Saluk, J.; Antosik, A.; Ponczek, M.B.; Żbikowska, H.M.; Borowiecka, M.; Nowak, P. Aronia melanocarpa as a protector against nitration of fibrinogen. Int. J. Biol. Macromol. 2013, 55, 264–268. [Google Scholar] [CrossRef]
- Bijak, M.; Nowak, P.; Borowiecka, M.; Ponczek, M.B.; Żbikowska, H.M.; Wachowicz, B. Protective effects of (-)-epicatechin against nitrative modifications of fibrinogen. Thromb. Res. 2012, 130, 123–128. [Google Scholar] [CrossRef]
- Hoppe, H.A. Taschenbuch der Drogenkunde; De Gruyter: New York, NY, USA, 1981. [Google Scholar]
- Berger, F. Handbuch der Drogenkunde, 1st ed.; Maudrich: Wien, Austria, 1949. [Google Scholar]
- Wawrzyniak, E. Leczenie Ziołami: Kompendium Fitoterapii; Contrast: Warszawa, Poland, 1992. [Google Scholar]
- Blumenthal, M.; Busse, W.R. The Complete German Commission E monographs: Therapeutic Guide to Herbal Medicines; The American Botanical Council: Austin, TX, USA, 1998. [Google Scholar]
- Marchelak, A.; Owczarek, A.; Matczak, M.; Pawlak, A.; Kolodziejczyk-Czepas, J.; Nowak, P.; Olszewska, M.A. Bioactivity potential of Prunus spinosa flower extracts: Phytochemical profiling, cellular safety, pro-inflammatory enzymes inhibition and protective effects against oxidative stress in vitro. Front. Pharmacol. 2017, 8, 680:1–680:15. [Google Scholar] [CrossRef] [Green Version]
- Marchelak, A.; Owczarek, A.; Rutkowska, M.; Michel, P.; Kolodziejczyk-Czepas, J.; Nowak, P.; Olszewska, M.A. New insights into antioxidant activity of Prunus spinosa flowers: Extracts, model polyphenols and their phenolic metabolites in plasma towards multiple in vivo-relevant oxidants. Phytochem. Lett. 2019, 30, 288–295. [Google Scholar] [CrossRef]
- Marchelak, A.; Olszewska, M.A.; Owczarek, A. Simultaneous quantification of thirty polyphenols in blackthorn flowers and dry extracts prepared thereof: HPLC-PDA method development and validation for quality control. J. Pharm. Biomed. 2020, 184, 113121:1–113121:8. [Google Scholar] [CrossRef] [PubMed]
- Marchelak, A.; Olszewska, M.A.; Owczarek, A. Data on the optimization and validation of HPLC-PDA method for quantification of thirty polyphenols in blackthorn flowers and dry extracts prepared thereof. Data Brief 2020, 29, 105319:1–105319:12. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [Green Version]
- Hollman, P.C.H. Absorption, bioavailability, and metabolism of flavonoids. Pharm. Biol. 2004, 42, 74–83. [Google Scholar] [CrossRef]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2019, 60, 626–659. [Google Scholar] [CrossRef] [PubMed]
- Serreli, G.; Deiana, M. In vivo formed metabolites of polyphenols and their biological efficacy. Food Funct 2019, 10, 6999–7021. [Google Scholar] [CrossRef]
- Olszewska, M.; Wolbiś, M. Flavonoids from the flowers of Prunus spinosa L. Acta Pol. Pharm. 2001, 58, 367–372. [Google Scholar]
- Olszewska, M.; Wolbiś, M. Further flavonoids from the flowers of Prunus spinosa L. Acta Pol. Pharm. 2002, 59, 133–137. [Google Scholar]
- Pryor, W.A.; Cueto, R.; Jin, X.; Koppenol, W.H.; Ngu-Schwemlein, M.; Squadrito, G.L.; Uppu, P.L.; Uppu, R.M. A practical method for preparing peroxynitrite solutions of low ionic strength and free of hydrogen peroxide. Free Radic. Biol. Med. 1995, 18, 75–83. [Google Scholar] [CrossRef]
- Kolodziejczyk-Czepas, J.; Wachowicz, B.; Moniuszko-Szajwaj, B.; Kowalska, I.; Oleszek, W.; Stochmal, A. Antioxidative effects of extracts from Trifolium species on blood platelets exposed to oxidative stress. J. Physiol. Biochem. 2013, 69, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Ghisaidoobe, A.B.T.; Chung, S.J. Intrinsic tryptophan fluorescence in the detection and analysis of proteins: A focus on Förster Resonance Energy Transfer techniques. Int. J. Mol. Sci. 2014, 15, 22518–22538. [Google Scholar] [CrossRef] [PubMed]
- Lettieri-Barbato, D.; Tomei, F.; Sancini, A.; Morabito, G.; Serafini, M. Effect of plant foods and beverages on plasma non-enzymatic antioxidant capacity in human subjects: A meta-analysis. Br. J. Nutr. 2013, 109, 1544–1556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szabó, C.; Ischiropoulos, H.; Radi, R. Peroxynitrite: Biochemistry, pathophysiology and development of therapeutics. Nat. Rev. Drug Discov. 2007, 6, 662–680. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomson, L. 3-nitrotyrosine modified proteins in atherosclerosis. Dis. Markers 2015, 2015, 708282:1–708282:8. [Google Scholar] [CrossRef]
- Frijhoff, J.; Winyard, P.G.; Zarkovic, N.; Davies, S.S.; Stocker, R.; Cheng, D.; Knight, A.R.; Taylor, E.L.; Oettrich, J.; Ruskovska, T.; et al. Clinical relevance of biomarkers of oxidative stress. Antioxid. Redox Signal. 2015, 23, 1144–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beckmann, J.S.; Chen, J.; Ischiropoulos, H.; Crow, J.P. Oxidative chemistry of peroxynitrite. Methods Enzymol. 1994, 233, 229–240. [Google Scholar]
- Miao, J.L.; Wang, W.F.; Pan, J.X.; Lu, C.Y.; Li, R.Q.; Yao, S.D. The scavenging reactions of nitrogen dioxide radical and carbonate radical by tea polyphenol derivatives: A pulse radiolysis study. Radiat. Phys. Chem. 2001, 60, 163–168. [Google Scholar] [CrossRef]
- Koenig, W. Haemostatic risk factors for cardiovascular diseases. Eur. Heart J. 1998, 19, 39–43. [Google Scholar]
- Lefevre, M.; Kris-Etherton, P.M.; Zhao, G.; Tracy, R.P. Dietary fatty acids, hemostasis, and cardiovascular disease risk. J. Am. Diet. Assoc. 2004, 104, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Stikarová, J.; Suttnar, J.; Sovova, Z.; Ceznerova, E.; Novak, J.; Louzil, J.; Kotlín, R.; Roselova, P.; Kaufmanova, J.; Chrastinova, L.; et al. Oxidative modified fibrinogen in cardiovascular diseases. Blood 2018, 132, 5011. [Google Scholar] [CrossRef]
- Mosesson, M.W. Fibrinogen and fibrin structure and functions. J. Thromb. Haemost. 2005, 3, 1894–1904. [Google Scholar] [CrossRef] [PubMed]
- Becatti, M.; Marcucci, R.; Bruschi, G.; Taddei, N.; Bani, D.; Gori, A.M.; Giusti, B.; Gensini, G.F.; Abbate, R.; Fiorillo, C. Oxidative modification of fibrinogen is associated with altered function and structure in the subacute phase of myocardial infarction. Arter. Thromb. Vasc. Biol. 2014, 34, 1355–1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehrenshaft, M.; Deterding, L.J.; Mason, R.P. Tripping up Trp: Modification of protein tryptophan residues by reactive oxygen species, modes of detection, and biological consequences. Free Radic. Biol. Med. 2015, 89, 220–228. [Google Scholar] [CrossRef] [Green Version]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Rutkowska, M.; Olszewska, M.A.; Kolodziejczyk-Czepas, J.; Nowak, P.; Owczarek, A. Sorbus domestica leaf extracts and their activity markers: Antioxidant potential and synergy effects in scavenging assays of multiple oxidants. Molecules 2019, 24, 2289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kicel, A.; Owczarek, A.; Kapusta, P.; Kolodziejczyk-Czepas, J.; Olszewska, M.A. Contribution of individual polyphenols to antioxidant activity of Cotoneaster bullatus and Cotoneaster zabelii leaves-structural relationships, synergy effects and application for quality control. Antioxidants 2020, 9, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Xia, T.; Duan, W.; Zhang, Z.; Li, Y.; Fang, B.; Xia, M.; Wang, M. Effects of organic acids, amino acids and phenolic compounds on antioxidant characteristic of Zhenjiang aromatic vinegar. Molecules 2019, 24, 3799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dabeek, W.M.; Marra, M.V. Dietary quercetin and kaempferol: Bioavailability and potential cardiovascular-related bioactivity in humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olthof, M.R.; Hollman, P.C.H.; Vree, T.B.; Katan, M.B. Bioavailabilities of quercetin-3-glucoside and quercetin-4’-glucoside do not differ in humans. J. Nutr. 2000, 130, 1200–1203. [Google Scholar] [CrossRef] [PubMed]
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of dietary polyphenols and gut microbiota metabolism: Antimicrobial properties. Biomed. Res. Int. 2015, 2015, 905215:1–905215:18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ou, K.; Gu, L. Absorption and metabolism of proanthocyanidins. J. Funct. Foods 2014, 7, 43–53. [Google Scholar] [CrossRef]
- Tomas-Barberan, F.; García-Villalba, R.; Quartieri, A.; Raimondi, S.; Amaretti, A.; Leonardi, A.; Rossi, M. In vitro transformation of chlorogenic acid by human gut microbiota. Mol. Nutr. Food Res. 2014, 58, 1122–1131. [Google Scholar] [CrossRef]
- Stalmach, A.; Williamson, G.; Crozier, A. Impact of dose on the bioavailability of coffee chlorogenic acids in humans. Food Funct. 2014, 5, 1727–1737. [Google Scholar] [CrossRef]
- Barrita, J.L.S.; Sánchez, M.S.S. Antioxidant role of ascorbic acid and his protective effects on chronic diseases. In Oxidative Stress and Chronic Degenerative Diseases—A Role for Antioxidants; Morales-Gonzalez, J.A., Ed.; IntTech Publisher: London, UK, 2013; pp. 449–484. [Google Scholar]
- German Nutrition Society (DGE). New reference values for vitamin C intake. Ann. Nutr. Metab. 2015, 67, 13–20. [Google Scholar] [CrossRef] [PubMed]









| Phytochemical Content | MED | DEF | EAF | References |
|---|---|---|---|---|
| TPC (mg GAE/g dw) | 206.07 ± 10.86 a | 464.57 ± 20.57 b | 584.07 ± 12.98 c | [18] |
| TFC (mg/g dw) | 125.12 ± 0.55 a | 490.63 ± 8.16 c | 325.53 ± 4.23 b | [18] |
| TPA (mg CYE/g dw) | 45.13 ± 2.38 a | 49.5 ± 2.23 a | 109.43 ± 3.71 b | [18] |
| TAC (mg/g dw) | 29.24 ± 0.76 c | 8.76 ± 0.27 a | 17.20 ± 0.47 b | [18] |
| TPH (mg/g dw) | 157.47 a | 491.69 c | 353.07 b | [20,21] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchelak, A.; Kolodziejczyk-Czepas, J.; Wasielewska, P.; Nowak, P.; Olszewska, M.A. The Effects of Prunus spinosa L. Flower Extracts, Model Polyphenols and Phenolic Metabolites on Oxidative/Nitrative Modifications of Human Plasma Components with Particular Emphasis on Fibrinogen In Vitro. Antioxidants 2021, 10, 581. https://doi.org/10.3390/antiox10040581
Marchelak A, Kolodziejczyk-Czepas J, Wasielewska P, Nowak P, Olszewska MA. The Effects of Prunus spinosa L. Flower Extracts, Model Polyphenols and Phenolic Metabolites on Oxidative/Nitrative Modifications of Human Plasma Components with Particular Emphasis on Fibrinogen In Vitro. Antioxidants. 2021; 10(4):581. https://doi.org/10.3390/antiox10040581
Chicago/Turabian StyleMarchelak, Anna, Joanna Kolodziejczyk-Czepas, Paulina Wasielewska, Pawel Nowak, and Monika A. Olszewska. 2021. "The Effects of Prunus spinosa L. Flower Extracts, Model Polyphenols and Phenolic Metabolites on Oxidative/Nitrative Modifications of Human Plasma Components with Particular Emphasis on Fibrinogen In Vitro" Antioxidants 10, no. 4: 581. https://doi.org/10.3390/antiox10040581